The recent outbreak of a deadly and highly contagious strain of mpox (monkeypox) in the Democratic Republic of the Congo (DRC) has raised concern among World Health Organization (WHO) and scientists. Clade I mpox variant causing the outbreak in the DRC has the potential to become a more serious outbreak due to its high mortality rate, rapid transmission and potential for international spread. Laboratory tests to detect Monkeypox are crucial in controlling the spread of the disease and providing rapid treatment to patients.
Current Situation of Monkeypox Cases
DRC is facing a major outbreak of Monkeypox due to a novel strain of clade I monkeypox virus (MPXV). DRC has seen more than 11,000 cases reported this year and 445 deaths. The WHO stated that there is a risk of cross-border and international spread risk since this more dangerous strain is more transmissible and has higher lethality. Currently, the mortality rate is about 5% in adults and 10% in children, with a high miscarriage rate among women.
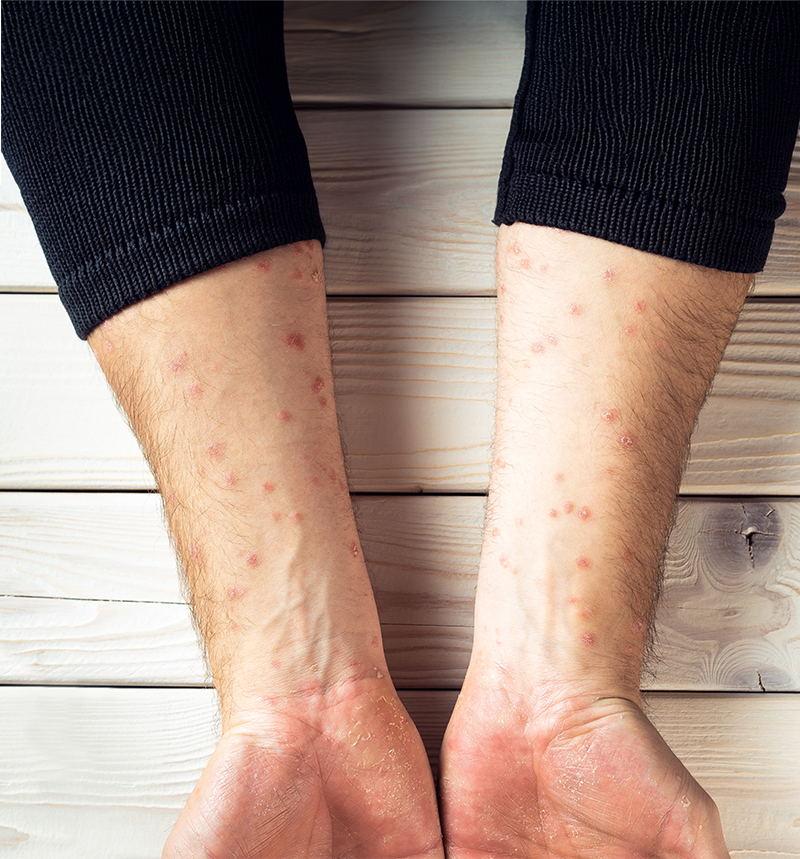
A couple of worrying observations have been since made after WHO declared a public health emergency. Firstly, Monkeypox is now spreading from person to person faster therefore this sustained transmission enables the virus to mutate also much faster. Secondly, there is no licenced Monkeypox-specific vaccine to prevent infections or specific anti-viral drugs available to treat clinically vulnerable people who can get severely affected by this disease.
Two years ago in 2022, another Monkeypox outbreak spread through Europe and the US prompting the WHO to declare a public health emergency caused by clade II of the virus which was controlled by vaccination of vulnerable groups
What is Monkeypox?
Monkeypox is a viral illness caused by the monkeypox virus, a species of the genus Orthopoxvirus and with two different clades: clade I and clade II. The virus causes lesions across the whole body which can last 2-4 weeks accompanied by fever, headache, muscle aches, back pain, low energy, and swollen lymph nodes.
Detection of Monkeypox
Laboratory confirmation of Monkeypox is usually done by testing skin lesion material by PCR. These tests are key to distinguishing Monkeypox from chickenpox, measles or other sexually transmitted diseases and for people to get treatment as early as possible and prevent further spread.
Bosphore Monkeypox Detection Kit v2, produced by Anatolia Geneworks, is one of these Real-Time PCR kits, that has been designed to detect the Monkeypox virus in human biological samples including lesion swabs, whole blood, serum, throat swabs, and saliva. Recommended biological specimens for laboratory diagnosis of Monkeypox are skin lesion material, including swabs of lesion surface and/or exudate, roofs from multiple lesions, or lesion crusts. This kit is integrated with both exogenous and endogenous integral control to check extraction, PCR inhibition, sampling or application errors.




